Abstract
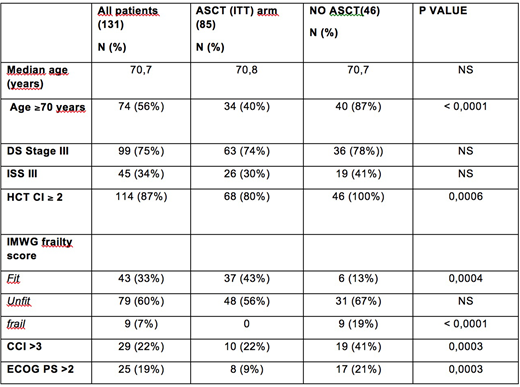
Introduction: Autologous stem cell transplantation (ASCT) has proven effective in Multiple Myeloma (MM) patients (pts) aged <65. However in several studies ASCT has proven safe and effective also in selected MM pts aged >65. With the increase of treatment options, several geriatric assessment tools have been proposed to help physicians in selecting treatment of appropriate intensity. However specific criteria for evaluating ASCT eligibility in elderly MM pts have been seldom investigated.Patients and Methods: From January 2013 to December 2017 131 consecutive newly diagnosed symptomatic MM pts aged 65-75 (M/F: 66/65) were considered for ASCT at our center according to physician's clinical judgement. The variables included in Charlson Comorbidity Index (CCI), Hematopoietic cell transplantation comorbidity index (HCT-CI), and International Myeloma Working Group (IMWG) frailty score were not considered for selection but were retrieved for this study and pts were classified accordingly. Of note, ADL and IADL were referred to pts status prior the occurrence of MM related symptoms. The impact of age and the predictive role of the above mentioned scores on progression-free survival (PFS) of pts selected or not for ASCT was analyzed. Pts characteristics are shown in Table 1. Results: Of 131 pts, 85 (65%) were judged transplant eligible (ASCT ITT arm) by the clinician and received bortezomib-based induction (VTD 94%, VD 5%, VCD 1%); 72 of them (85%) actually underwent ASCT (70% single, 30% double) with melphalan conditioning 200 mg/sqm in 68% of cases. The 46 pts considered ineligible to ASCT received a less intensive first line treatment (89% VMP, 4% Daratumumab-VMP, 2% MP, 5% steroids/palliation). Complete remission (CR) after the first ASCT was higher in the ASCT (ITT) vs the NO ASCT group (43,5% vs 26%, respectively; p 0.048), whereas ORR and ³VGPR rates were comparable (83% vs 74% and 76,4% vs 61%, respectively). Transplant related mortality (TRM) was 0%. 2 year death rate was 13% in the ASCT (ITT) arm and was due to PD in 91% of cases (the latter 9% concerns a cardiac arrest secondary to aspiration pneumonia in a pt in VGPR 3 months after first ASCT). After a median follow-up of 27 months, PFS was 35,6 in the ASCT (ITT) group vs 19,9 months in NO ASCT pts, respectively; p 0,013, HR 0,42 (95% CI: 0,25-0,71). HCT-CI was ≥2 in 87% of pts overall. PFS was better in pts with HCT-CI 0-1 compared to HCT≥2 (NR vs 27,3 months; p 0,025, HR 0,45, 95% CI 0,23-0,91) and also in pts with HCT-CI ³2 undergoing ASCT (ITT) than in NO ASCT pts: median 34 vs 19,9 months, p 0,008 (HR 0,5, 95%CI 0,30-0,84). CCI was 0-3 in 78% of pts. Their outcome according was similar to pts with CCI >3 but ASCT performed better than NO ASCT also in the few (median PFS 51,4 vs 16,9 months; p 0,04, HR 0,37, 95%CI 0,15-0,95). IMWG frailty score was more useful. No pts classified as FRAIL had been considered eligible to ASCT by clinical decision with a significantly worse outcome compared to FIT and UNFIT pts (median PFS: 7,9 months vs 32,9 and 29,6; FIT vs FRAIL: p < 0.0001, HR 0,02, 95%CI 0,004-0,008; UNFIT vs FRAIL: p < 0,001, HR 0,03 95%CI 0,007-0,12). Unlike HCT-CI and CCI, the distribution of patients according to IMWG score was more balanced both overall and between the ASCT (ITT) and NO ASCT groups, particularly for UNFIT pts. However on the whole no outcome differences were observed between FIT and UNFIT pts. PFS was better in FIT&UNFIT pts in the ASCT (ITT) group than in the NO ASCT group: median 35,6 months vs 25,8 months; p 0,04, HR 0,54, 95%CI 0,31-0,97. However, in the ASCT (ITT) group, the age group 65-69 years fared better than pts ³70 years (51,5 vs 27,7 months, p 0,0037; HR 0.34, 95% CI 0.17-0.7). Indeed, in IMWG UNFIT patients aged ³70, the PFS of the ASCT (ITT) group was comparable to NO ASCT group (18 vs 27 months, p 0,33) whereas in UNFIT pts aged 65-69, PFS was superior in pts treated more intensively by physician choice: 43,3 months in ASCT (ITT) arm vs 18,4 months in NO ASCT (p 0.01, HR 0.03; 95%CI 0.003-0.24). Conclusion: In an unselected series of elderly MM pts aged 65-75 and undergoing ASCT according to clinical judgement the outcome of ASCT pts was better than those of NO ASCT. CCI and HCT-CI score proved of little help for better identifying the best candidates to ASCT. Conversely the IMWG frailty score would be of help in identifying the category of UNFIT pts aged 70-75 whose outcome with ASCT selected by clinical judgement was no better than with less intensive treatments.
Table 1:
Belotti:Amgen: Other: Advisory Board; Celgene: Other: Advisory Board. Cattaneo:GILEAD: Other: Advisory Board. Rossi:TEVA: Other: ADVISORY BOARD; CELGENE: Other: ADVISORY BOARD; SANOFI: Other: ADVISORY BOARD; MUNDIPHARMA: Honoraria; ABBVIE: Other: ADVISORY BOARD; JANNSEN: Other; PFIZER: Other: ADVISORY BOARD; ROCHE: Other: Advisory Board; NOVARTIS: Honoraria; SANDOZ: Honoraria; GILEAD: Other: ADVISORY BOARD; BMS: Honoraria; JAZZ: Other: ADVISORY BOARD; AMGEN: Other: ADVISORY BOARD.
Author notes
Asterisk with author names denotes non-ASH members.